709
Views & Citations10
Likes & Shares
Cerebral ischemia is a devastating of the
brain without regenerative treatment options, demanding a vigorous search for
new therapeutic strategies. Despite the initial hope that cell-based therapies
may stimulate restorative processes in the ischemic brain, it is now recognized
that aging processes may promote generate an unfavorable environment for such
treatments. By this project we take advantage of our previous experience on
stroke therapies and aim at developing of a novel stem cell-seeded hydrogel to
support the recovery of brain structure and function after stroke by
exploting new developments in the stem cell biology and nourishing hydrogels
for cell culture of neuronal cells. Human stroke data suggests that the
cortical ischemic core but not the penumbra is a determinant of clinical
outcomes after acute ischemic stroke. Therefore any therapeutic delivered to
the cavity will have direct access to the tissue target for repair and
recovery. Recent advances in tissue engineering have developed injectable
hydrogels that can provide both a mechanical support and trophic factors for
neuronal precursor cells (NPCs). Since stroke affects mostly the elderly, it is
highly desirable and clinically important to test the efficacy of cell
therapies in aged brain microenvironments. Recently new technologies to promote
regeneration in the damaged brain area have been developed by embedding stem
cells in nourishing hydrogels, thereby recreating a neurovascular niche by
recruitment of neural precursor cells and microvascular cells. Through this
novel experimental technique ones expect a significant improvement in tissue
integrity and functional restoration after stroke. Given the overwhelming
importance of stroke therapy for both patients and society, this approach, if
successful, will be a breakthrough in the field.
Key words: stroke; therapy; stem cells; hydrogels;
recovery
INTRODUCTION
Stroke has limited treatment options, demanding a
vigorous search for new therapeutic strategies. Despite the initial hope that
cell-based therapies may stimulate restorative processes in the ischemic brain,
it is now recognized that aging processes may promote generate an unfavourable
environment for such treatments. Old age is associated with an enhanced
susceptibility to stroke and aged animals, recover poorly from brain injuries
compared to young. Since stroke affects mostly the elderly, it is highly desirable
and clinically important to test the efficacy of cell therapies in aged brain
microenvironments. We have shown that the aged rat brain is not refractory to
cell-based therapy as previously thought, and that it also supports plasticity
and remodelling. Yet, important differences exist in the aged compared with young
brain, i.e., the accelerated progression of ischemic injury to brain
infarction, the reduced rate of endogenous neurogenesis and the delayed
initiation of neurological-recovery. These age-related aspects should be
carefully considered in the clinical translation of restorative therapies. We found that at genetic and
cellular level there are significant differences in behavioral, cytological and
genomics responses to injury in old animals as compared with the young ones [Popa-Wagner A et al., 2011; Buga AM et al., 2013]. Behaviorally, the aged rats have the capacity
to recover after cortical infarcts albeit to a lower extent than the younger
counterparts. Similarly, the increased vulnerability of the aged brain to
stroke, together with a decreased interhemisphere synchrony after stroke,
assessed by different experimental methods (MRI, fMRI, in vivo microscopy, EEG)
leads to unfavorable recovery of physical and cognitive functions in aged
people and may have a prognostic value for the recovery of stroke patients.
Furthermore, in elderly, comorbidities like diabetes or arterial hypertension
are associated with higher risk of stroke, increased mortality and disability,
and poorer functional status and quality of life. Aging brain reacts strongly
to ischemia–reperfusion injury with an early inflammatory response. The process
of cellular senescence can be an important additional contributor to chronic
post-stroke by creating a ‘‘primed’’ inflammatory environment in the brain.
Overall, these proinflammatory reactions promote early scar formation
associated with tissue fibrosis and reduce functional recovery. A better
understanding of molecular factors and signaling pathways underlying the
contribution of comorbidities to stroke-induced pathological sequelae, may be
translated into successful treatment or prevention therapies for age-associated
diseases which would improve lifespan and quality of life [Popa-Wagner A et al., 1998; Popa-Wagner A et al., 2006; Popa-Wagner A et al., 2011; Buga AM et al., 2013; Di Napoli M et al., 2012; Badan I et al., 2003;
Buchhold B et al., 2007].
After cortical stroke, a cavity and a bordering
scar to the perinfarct, develop. Contrary to a commonly held view that the scar
impairs neural
recovery and repair, we have shown that the poststroke scar is actually
vascularized fibrous tissue [Balseanu AT et al., 2014]. This finding suggests
that astrocytic scar formation is not a principal obstacle to the re-growth of
injured axons across severe CNS lesions, and that scar-forming astrocytes may
actually support the regeneration of appropriately stimulated CNS axons [Buga
AM et al., 2014; Buga AM et al., 2015; Anderson MA et al., 2016]. Moreover, any therapeutic delivered to the cavity will have direct
access to the tissue target for
repair and recovery.
Many vascular endothelial cells and neurovascular structures in the core
survive from the ischemic insult and regenerative activities such as
proliferation of endothelial cells and formation/invasion of neural progenitor
cells takes place in the core many days after stroke. As a result, extensive
neurovascular networks are established in the ischemic core 14 days after
stroke [Popa-Wagner et al., 2007; Buga AM et al., 2014]. Although
the surviving cells in the core after stroke are few and the neurovascular
structures may be imperfect or immature, they could provide a minimum but vital
infrastructure for possible regeneration from endogenous mechanisms. For
regenerative therapies using exogenous stem cells and neural progenitors, our
data suggest that the microenvironment of ischemic core several days after
stroke provides certain cellular and strong trophic supports for cells to
survive while the remaining and regenerating neurovascular infrastructure may
be utilized for repair of damaged neural networks.
Attractive therapeutic strategies stimulating and finally enhancing the
natural post-stroke regeneration process include methods of training such as
physio- or rehabilitative therapy or methods of cellular therapy [Hermann DM
et al., 2012; Honmou O
et al., 2012; Popa-Wagner
A et al., 2014]. Stroke
induces a specific remodeling of the brain vasculature. Using an aged rat
model of stroke, we previously found that at two weeks after stroke the
microvascular density was reduced in aged rats as compared to young animals on
a background of persistent
upregulation of genes coding for matrix proteases and inflammatory mediators. However,
beyond the inhibitory fibrotic scar, in a region made of soft tissue that we
dubbed “islet of regeneration”, the vascular density was similar in the two age
groups. Unlike in rats, the post-stroke angiogenesis in human patients is
vigorous at one week post-stroke, and correlates well with the post-stroke
survival time. By comparative transcriptomics of angiogenesis we identified 36
new stroke-related genes some of which may be used as new therapeutic targets
that may help redress the dysregulation of angiogenesis in the
infarcted area of aged brain. We also found that the aged human brain is
capable of mounting a vigorous angiogenic response after stroke, which most
likely reflects the remaining brain plasticity of the aged brain [Buga AM et
al., 2014; Raluca Elena Sandu et al., 2016].
We were also concerned with identifying differences in gene expression between the
young and the aged brain after a lesion such as stroke. To this end, we employed proteomics and the
Affymetrix platform to analyze the whole-gene transcriptome following temporary
ligation of the middle cerebral artery in aged and young rats. The
correspondence, heat map, and dendrogram analyses independently suggest a
differential, age-group-specific behaviour of major gene clusters after stroke.
Overall, the pattern of gene expression strongly suggests that the response of
the aged rat brain is qualitatively rather than quantitatively different from
the young, i.e. the total number of regulated genes is comparable in the two
age groups, but the aged rats had great difficulty in mounting a timely
response to stroke. Our study indicates that four genes related to neuropathic
syndrome, stress, anxiety disorders and depression (Acvr1c, Cort, Htr2b and
Pnoc) may have impaired response to stroke in aged rats. New therapeutic
options in aged rats may also include Calcrl, Cyp11b1, Prcp, Cebpa, Cfd, Gpnmb,
Fcgr2b, Fcgr3a, Tnfrsf26, Adam 17 and Mmp14. An unexpected target is the enzyme
3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 in aged rats, a key enzyme in
the cholesterol synthesis pathway. Post-stroke axonal growth was compromised in
both age groups. Our results suggest that a multi-stage, multimodal treatment
in aged animals may be more likely to produce positive results. Such a
therapeutic approach should be focused on tissue restoration but should also
address other aspects of patient post-stroke therapy such as neuropathic
syndrome, stress, anxiety disorders, depression, neurotransmission and blood
pressure [Junker H et al., 2007; Buga AM et al., 2008; Buga AM et al., 2012; Joseph C et al., 2012; Buga AM et al., 2014].
Although the surviving cells in the core after stroke are few and the
neurovascular structures may be imperfect or immature, they could provide a
minimum but vital infrastructure for possible regeneration from endogenous
mechanisms. For regenerative therapies using exogenous stem cells and neural
progenitors, our data suggest that the microenvironment of ischemic core
several days after stroke provides certain cellular and strong trophic supports
for cells to survive while the remaining and regenerating neurovascular
infrastructure may be utilized for repair of damaged neural networks.
Attractive therapeutic strategies to enhance
post-stroke recovery of aged brains include methods of cellular therapy that
can enhance the endogenous restorative mechanisms of the injured brain. Since
stroke afflicts mostly the elderly, it is highly desirable to test the efficacy
of cell therapy in the microenvironment of aged brains that is generally
refractory to regeneration. In particular, stem cells from the bone marrow
allow an autologous transplantation approach that can be translated in the near
future to the clinical practice. Such a bone marrow-derived therapy includes
the grafting of stem cells as well as the delayed induction of endogenous stem
cell mobilisation and homing by the stem cell mobiliser Granulocyte-colony
Stimulating Factor (G-CSF). In previous work, we tested the hypothesis that
grafting of bone marrow-derived pre-differentiated mesenchymal cells (BM MSCs)
in G-CSF-treated animals improves the long-term functional outcome in aged
rodents. To this end, G-CSF alone (50 µg/kg) or in combination with a single
dose (106 cells) of rat BM MSCs were administered intravenously to
Sprague-Dawley rats at six hour safter transient occlusion (90 min) of the
middle cerebral artery. Infarct volume was measured by MRI at 3 and 48 days
post-stroke and additionally by immunhistochemistry at day 56. Functional
recovery was tested during the entire post-stroke survival period of 56 days.
Daily treatment for post-stroke aged rats with G-CSF led to a robust and
consistent improvement of neurological function after 28 days. The combination
therapy also led to robust angiogenesis in the formerly infarct core and beyond
in the “islet of regeneration”. However, G-CSF + BM MSCs may not impact at all
on the spatial reference-memory task or infarct volume and therefore did not
further improve the post-stroke recovery. We suggest that in a real clinical
practice involving older post-stroke patients, successful regenerative
therapies would have to be carried out for a much longer time [Balseanu AT
et al., 2014; Buga AM et al., 2015].
Recent advances in tissue engineering have
produced hydrogels have been designed to promote stem cell survival, minimize
wound scar formation, and enhance stem cell engraftment [Potter et al., 2008;
Chai et al., 2007]. Hydrogels for CNS applications are easily transplanted into
the adult brain without damage and support survival and differentiation of
stem/progenitor cells in vitro and in vivo. Further, very important, hyaluronan
gels have mechanical properties similar to brain tissue and do not promote local
scarring or tissue reaction These gels influence neural differentiation and
allow neuronal sprouting and ingrowth into the gel [Van Wie BJ et al., 2007;
Zhong J et al., 2010; Nih et al., 2016].
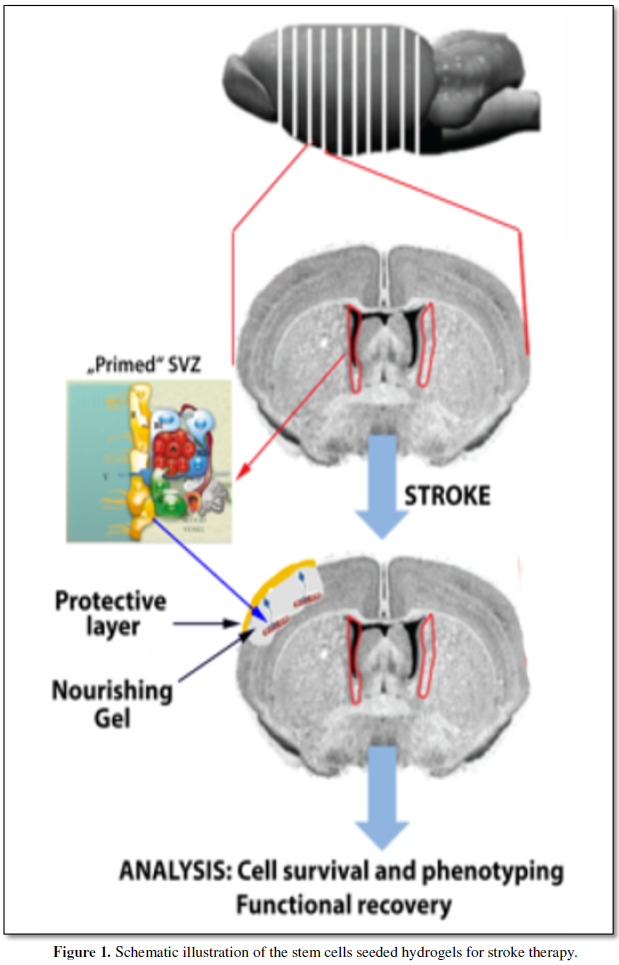
Finally, the strole cavity containing the embedded SVZ will by sealed by a protective layer containing microvascular endothelial cells and dermal fibroblasts consisting of a collagen-GAG, three-dimensional matrix colonized by human dermal fibroblasts [Froget S et al., 2003].
- Popa-Wagner A, Buga AM and Kokaia Z. Perturbed Cellular Response to Brain Injury During Aging. Aging
Research Reviews, 2011; 10(1):71-79. doi: 10.1016/j.arr.2009.10.008.
- Buga AM, Di Napoli M, Popa-Wagner A. Preclinical models of stroke in
aged animals with or without comorbidities: role of neuroinflammation. Biogerontology,
2013; 10(1):71-79. doi: 10.1016/j.arr.2009.10.008.
- Popa-Wagner A, Schröder
E, Walker LC, Kessler C. Beta-Amyloid precursor protein and
ss-amyloid peptide immunoreactivity in the rat brain after middle cerebral
artery occlusion: effect of age. Stroke, 1998; 29(10):2196-202.
- Popa-Wagner A, Dinca S., Yalikun L. et al. (2006)
Accelerated delimitation of the infarct zone by capillary-derived
nestin-positive cells in aged rats. Current Neurovascular Research, 2006;
3: 3-13.
- Di Napoli M, Godoy DA, Campi V, et al.
C-Reactive Protein After Intracerebral Hemorrhage. Time-course, Tissue
Localization and Prognosis. Neurology, 2012; 79:660-699.
- Badan I, Platt D, Kessler
C, Popa-Wagner A. Temporal dynamics of degenerative and regenerative
events associated with cerebral ischemia in aged rats.
Gerontology, 2003; 49(6):356-65. doi:10.1159/000073763
- Buchhold B, Mogoanta L, Suofu Y, et al.
Environmental enrichment improves functional and neuropathological indices
following stroke in young and aged rats. Restorative Neurol. Neurosci.
2007; 25: 1–18.
- Balseanu AT, Buga AM, Catalin
B, Wagner DC, Boltze J, Zagrean AM, Reymann
K, Schaebitz W, Popa-Wagner A. Multimodal Approaches for
Regenerative Stroke Therapies: Combination of Granulocyte
Colony-Stimulating Factor with Bone Marrow Mesenchymal Stem Cells is Not
Superior to G-CSF Alone. Front Aging Neurosci, 2014; Jun
23;6:130.
- Buga AM, Margaritescu C, Scholz CJ, et al. Transcriptomics of post-stroke angiogenesis in the aged brain. Front
Aging Neurosci., 2014; 6:44. doi:0.3389/fnagi.2014.00044.
- Buga AM, Scheibe J, Moller
K, Ciobanu O, Posel C, Boltze J, Popa-Wagner A.
Granulocyte colony-stimulating factor and bone marrow mononuclear cells
for stroke treatment in the aged brain. Curr Neurovasc Res, 2015;
12(2):155-62.
- Anderson
MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi
R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV.
Astrocyte scar formation aids central nervous system axon regeneration.
Nature, 2016; Apr 14;532(7598):195-200. doi: 10.1038/nature17623
- Popa-Wagner
A, Badan I, Walker L, et al. Accelerated infarct development,
cytogenesis and apoptosis following transient cerebral ischemia in aged
rats. Acta Neuropathol. (Berlin), 2007; 113:277-293.
- Hermann DM, Chopp M.
Promoting brain remodelling and plasticity for stroke recovery:
therapeutic promise and potential pitfalls of clinical translation. Lancet
Neurol, 2012; Apr;11(4):369-80. doi:
10.1016/S1474-4422(12)70039-X
- Honmou O, Onodera
R, Sasaki M, Waxman SG, Kocsis JD. Mesenchymal stem cells:
therapeutic outlook for stroke. Trends Mol
Med, 2012; May;18(5):292-7. doi: 10.1016/j.molmed.2012.02.003
- Popa-Wagner
A, Buga AM, Doeppner TR, Hermann DM. Stem
cell therapies in preclinical models of stroke associated with aging.
Front Cell Neurosci, 2014; Nov 3;8:347. doi:
10.3389/fncel.2014.00347.
- Junker
H, Suofu Y, Venz S, Sascau M, Herndon JG, Kessler
C, Walther R, Popa-Wagner A. Proteomic identification of an
upregulated isoform of annexin A3 in the rat brain following reversible
cerebral ischemia. Glia, 2007; Dec;55(16):1630-7.
- Raluca
Elena Sandu, Ana-Maria Buga, Adrian Tudor Balseanu, et al. Twenty four
hours hypothermia has temporary efficacy in reducing brain infarction and
inflammation in aged rats. Neurobiology of Aging, 2016; 38:127-140.
- Junker
H, Suofu Y, Venz S, et al. Proteomic identification of an upregulated
isoform of Annexin A3 in the rat brain following reversible cerebral
ischemia. Glia, 2007; 55:1630-1637
- Buga AM,
Sascau M, Herndon JG, Kessler K et al. The genomic
response of the contralateral cortex to stroke is diminished in the aged
rats. J. Cell. Mol. Med., 2008; 12: 2731-2753.
- Buga AM, C
Scholz, S Kumar, et al. Identification of new therapeutic targets by
genome-wide analysis of gene expression in the ipsilateral cortex of aged
rats after stroke. PLoS ONE, 2012; 7: e50985.
- Potter
W, Kalil RE, Kao WJ. Biomimetic material systems for neural
progenitor cell-based therapy. Front Biosci, 2008; Jan 1;13:806-21.
- Chai
P, Napolitano AP, Dean DM, Morgan JR. Dynamics of the
self-assembly of complex cellular aggregates on micromolded nonadhesive
hydrogels. Tissue Eng, 2007; Aug;13(8):2087-94.
- Van
Wie BJ, Metallo CM, Mohr JC, Detzel CJ, de Pablo
JJ, Palecek SP. Engineering the stem cell microenvironment. Biotechnol
Prog, 2007; Jan-Feb;23(1):18-23.
- Nih
LR, Moshayedi P, Llorente IL, Berg AR, Cinkornpumin
J, Lowry WE, Segura T, Carmichael ST. Engineered HA
hydrogel for stem cell transplantation in the brain: Biocompatibility data
using a design of experiment approach. Data Brief, 2016; Nov
24;10:202-209.
- Joseph
C, Buga AM, Vintilescu R, Balseanu AT, Moldovan M, Junker H, Walker L,
Lotze M, Popa-Wagner A. Prolonged gaseous hypothermia prevents the
upregulation of phagocytosis-specific protein Annexin 1 and causes low-amplitude
EEG activity in the aged rat brain after cerebral ischemia. J Cereb Blood
Flow Metab, 2012; 32:1632-42.
- Balseanu AT, Buga
AM, Catalin B, Wagner DC, Boltze J, Zagrean
AM, Reymann K, Schaebitz W, Popa-Wagner A. Multimodal
Approaches for Regenerative Stroke Therapies: Combination of Granulocyte
Colony-Stimulating Factor with Bone Marrow Mesenchymal Stem Cells is Not
Superior to G-CSF Alone. Front Aging Neurosci, 2014; Jun
23;6:130.
- Zhong
J, Chan A, Morad L, et al. Hydrogel matrix to support stem cell survival
after brain transplantation in stroke. Neurorehabil Neural Repair, 2010;
24(7):636-44. doi: 10.1177/1545968310361958.
- Froget
S, Barthelemy E, Guillot F, et al. Wound healing mediator production by
human dermal fibroblasts grown within a collagen-GAG matrix for skin
repair in humans. Eur Cytokine Netw., 2003; 14(1):60-64.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Pathology and Toxicology Research
- International Journal of Diabetes (ISSN: 2644-3031)